What Happened
The Mississippi State Board of Medical Licensure (MSBML) and Mississippi Board of Pharmacy (MBP) recently issued an important joint statement regarding the procurement of controlled substances by healthcare providers practicing at Ambulatory Surgery Centers (ASCs). View a copy.
What it Means
ASCs in Mississippi that currently utilize a single practitioner’s Drug Enforcement Administration (DEA) permit to procure controlled substances for multiple providers must discontinue this practice and obtain: (1) an Outpatient Surgery Centers/Clinic pharmacy permit from the MBP; and (2) a Hospital/Clinic registration from the DEA. According to the Joint Statement, the DEA has agreed to delay inspections for noncompliance and enforcement of violations until April 1, 2023.
Mississippi Institutional Pharmacy Permits
The Joint Statement advises ASCs that presently do not have a state pharmacy permit, to obtain a pharmacy permit from the MBP before applying with the DEA. When applying for a state pharmacy permit ASCs have two options: (1) an Outpatient Surgery Centers/Clinic Pharmacy Services permit:
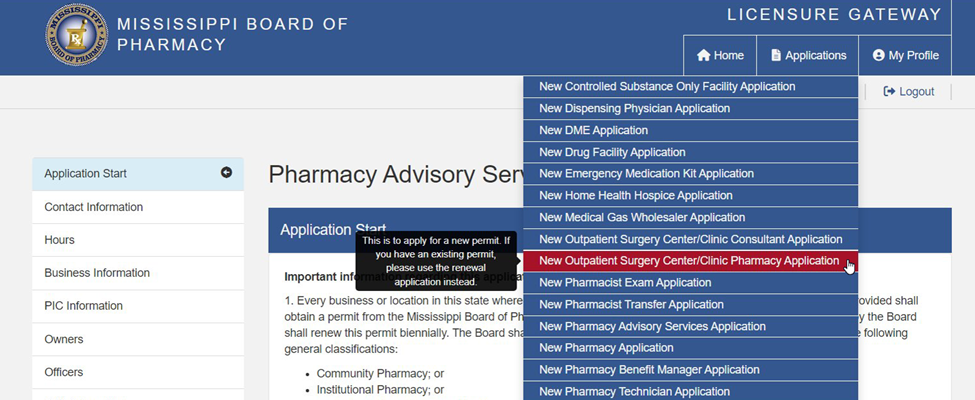
or (2) an Outpatient Surgery Centers/Clinic Consultant Permit.
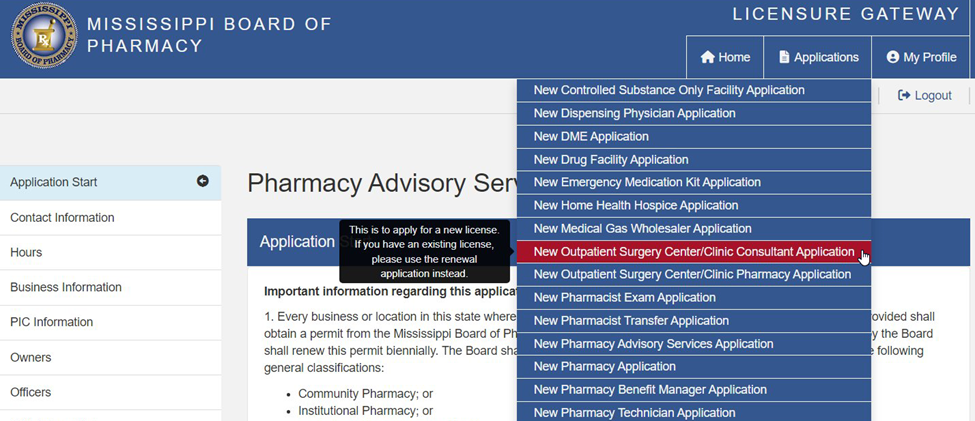
Applications for both types of permits can be found on the MBP’s Licensure Gateway website (MBP Licensure Gateway ).[1]
The following is a brief summary of these two permits:
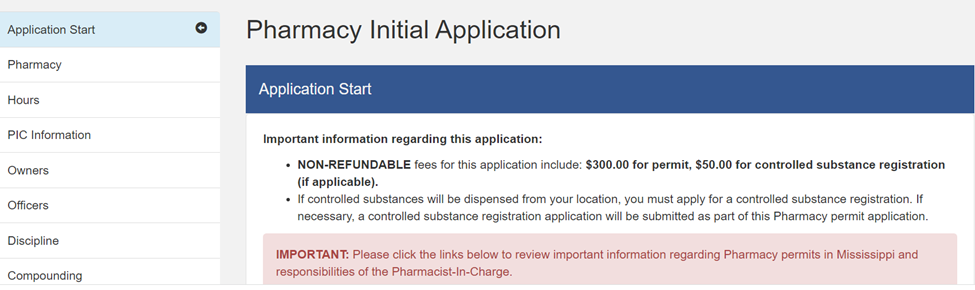
- Institutional I Pharmacy Permit: MBP regulations provide that “[e]very business or location in this state where prescription drugs are maintained and/or pharmacy services are provided shall obtain a permit as a pharmacy from the [MBP].”[2] ASCs (referred to as “outpatient surgery facilities”) fall under the MBP’s “Institutional I Pharmacy” designation.[3] ASCs applying for this standard permit use should use the “New Outpatient Surgery Center/Clinic Pharmacy Application” (MBP Form 13.3). The first page of this application in the Gateway looks like this:
- Consultant Permit: A “Consultant Pharmacist” is a “Mississippi licensed pharmacist who is responsible for developing, coordinating and supervising pharmaceutical services on a regularly scheduled basis in an institutional facility….”[4] The Joint Statement states that this must be “at least a monthly arrangement.” Consultant permitting falls under the general category of “Pharmacy Advisory Services” (MBP Form 13.4).[5]
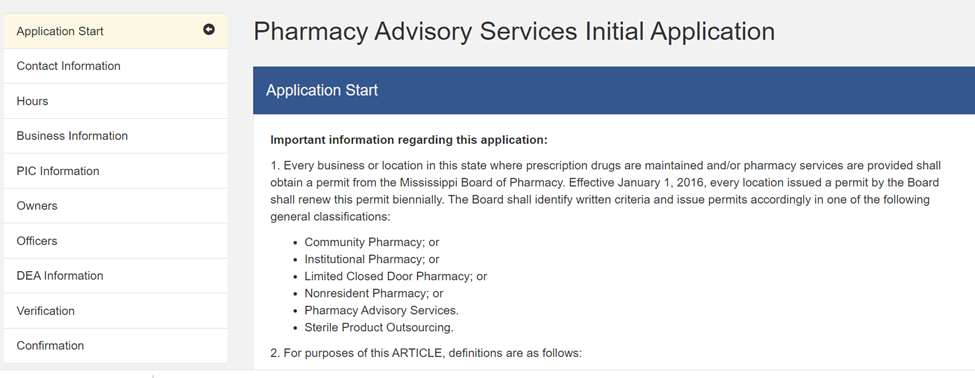
MBP regulations describe these types of services, when provided in an ASC (outpatient surgery) setting, as: “A pharmacist supervises appropriate documentation of administration, wastage and disposal of medications in accordance with documented policies and procedures of the facility. The Medical Director of the facility is responsible for obtaining the Drug Enforcement Administration (DEA) registration number for the facility and compliance with applicable DEA regulations.”[6]
DEA Guidance
The Joint Statement includes DEA guidance excerpts to assist ASCs presently utilizing individual practitioner registrations to transition to their own institutional DEA number.[7] Each applicant must fill out their own DEA Form 224, which can only be applied for online via the DEA’s website at DEA Diversion Forms.
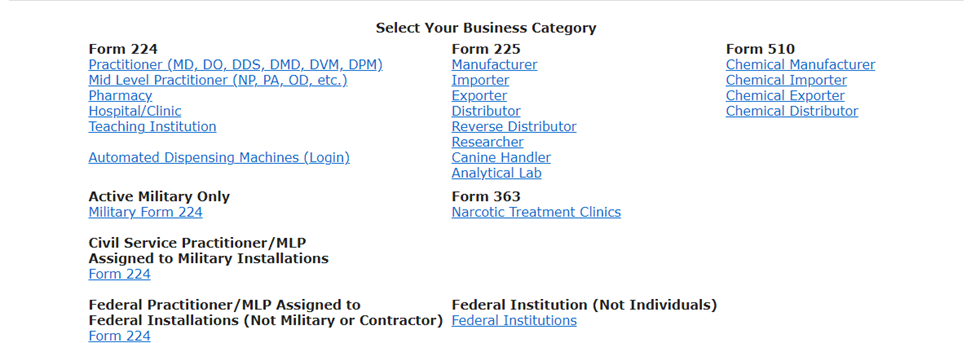
Once “New Application” is selected, ASC owners are directed by the DEA Guidance to select the Business Category of “Hospital/Clinic,” and will then proceed to enter information regarding their ASC’s general business information, activity and drug schedule information, state license information, and background information. When applying for a new registration applicants are directed to check “Yes” – to request DEA Form 222’s to purchase Schedule I and II controlled substances from suppliers.
After the application has been processed the ASC applicant may create a power of attorney for whomever they choose to be their representative to order controlled substances if the applicant—in most instances a corporate officer—wishes to delegate the responsibility to another.[8] Also, in order to “transfer and close out” the previously used individual practitioner number, the individual practitioner must take a final inventory[9] and transfer all controlled substances from the practitioner’s DEA number to the new institutional provider number. The ASC must also take an inventory under its new DEA number and receive all controlled substances from the individual practitioner’s DEA number.[10]
Finally, the individual practitioner DEA number that was previously used as the primary registration for the surgery center may be either: 1) retained at the current location for prescribing only; 2) moved to a different practice location to be used to prescribe, order, store, administer, or dispense controlled substances; or 3) discontinued by proper notification to the DEA.[11]
Butler Snow will continue to monitor and analyze these regulatory developments. Please contact any member of the firm’s Health Law practice group for further information.
[1] Users of the Gateway must create an online profile to register for the use of this official site.
[2] Miss. Bd. Pharmacy Regulations, Art. VI.1.
[3] Id. at 2.B.(1).
[4] Id. at XXX.A.2.
[5] Id. at VI.1.E.
[6] Id.
[7] An “institutional provider” is defined per DEA regulations as “a hospital or other person (other than an individual) licensed, registered, or otherwise permitted, by the United States or the jurisdiction in which it practices, to dispense a controlled substance in the course of professional practice, but does not include a pharmacy.” 21 C.F.R. §1300.01.
[8] See 21 C.F.R. § 1301.13(j); 21 C.F.R. § 1305.05. A sample power of attorney is set forth at § 1305.05(c).
[9] Schedules II and III-V are to be inventoried separately per the guidance.
[10] Records of the transfer of all controlled substances, including any applicable Form 222 for transferred Schedule II drugs and Schedule III-IV invoices are to be kept for a period of two years.
[11] The guidance directs that notification of the discontinuance be given in writing to angela.c.martin@dea.gov and that a DEA Certificate of Registration and unexecuted/voided DEA Form 222 be delivered to the DEA’s Jackson, Mississippi office at 100 W. Capitol Street, Suite 1100, Jackson, Mississippi 39296.
